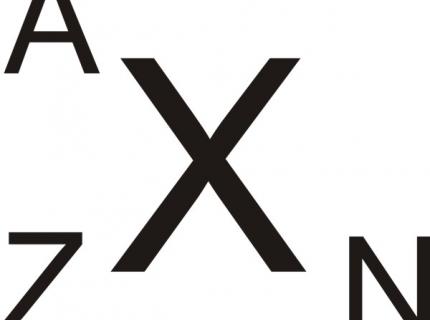Isotopes
Isotopes are atoms of one element (X) with the same number of protons (Z), but with a different number of neutrons (N). The sum of protons and neutrons presents the atom mass (A).
The 92 elements of the periodic table of the elements create more than 1000 isotopes. Isotopes are differentiated by 2 groups, stable and radioactive isotopes.
Isotopes are valuable and important for the determination
- of age (dating)
- of origin, genesis and development of rock, water, gases, and organic material or elements and compounds, respectively
- of the renewable carbon proportion
- of the radioactive charge.
Modern hydrogeology can not be imagined without isotope methods anymore. They provide a very detailed insight into the hydrological cycle. They are also an important tool for the optimisation of water management of aquifers and wells.
Measurements of the contents of environmental isotopes permit the study of hydrological systems in large regional and temporal scales. In particular isotope analyses allow the characterisation and quantification of groundwater components under difficult groundwater flow conditions.
Applied isotope systems in the hydrogeology are:
MEDIUM |
ISOTOPE SYSTEM |
INFORMATION |
| Water | 18O/16O (δ18O) and 2H/1H (δ2H) of H2O | Climatic conditions of groundwater recharge (altitude of catchment area, pleistocene recharge); Amount and flow time of bank infiltrates and precipitation discharge; evaporation processes; Identification of formation waters; water-rock-interaction; interaction with dissolved gases (e.g. CO2, VOCs) |
| Water | 3H of H2O | mean residence time of young groundwaters (< 60 a); amount of young groundwater components; evaluation of natural guardedness; identification of landfill leachate |
| Dissolved substances and gases | 34S/32S (δ34S) of SO4, H2S and 18O/16O (δ18O) of SO4 |
genesis and origin of dissolved sulphate and sulphur hydrogen; reduction and oxidation processes |
| Dissolved substances and gases | 13C/12C (δ13C) of DIC, CO2, DOC, CH4 | evaluation of carbon system, genesis, evolution and interaction of inorganic und organic carbon components |
| Dissolved substances and gases | 14C of DIC, CO2, DOC, CH4 | dating of old groundwater (thousands to ten thousands); evaluation of natural guardedness; origin and genesis of gases |
| Dissolved substances and gases | 15N/14N (δ15N) of NO3, NO2, NH4, N2 | evalution of the nitrogen system; origin and degradation processes of dissolved nitrogen components (denitrification) |
| Dissolved substances | 37Cl/35Cl (δ37Cl) of Cl- | genesis and origin of dissolved chloride; development of saline waters |
| Dissolved substances | 87Sr/86Sr | origin of groundwater; water-rock-interaction |
| Dissolved substances | 223Ra, 224Ra, 226Ra, 228Ra | radiological evaluation; change of inflow during pumping tests; water-rock-interaction |
| Dissolved substances | 234U, 235U,238U | radiological evaluation; water-rock-interaction |
| Dissolved substances | 134Cs,137Cs, 90Sr, 239Pt, 131I, 129I | influence of anthropogenic radionuclides (Tschernobyl, Fukushima, nuclear research center, medicine) |
| Dissolved gases | 85Kr | mean residence time of young groundwater (< 60 a); amount of young groundwater component |
| Dissolved gases | 81Kr | dating of very old groundwater (hundred thousands) |
| Dissolved gases | 3He/4He, 36Ar/40Ar, 20Ne,21Ne,22Ne | relative age of groundwater; water-rock-interaction; origin and development of water |
| Dissolved gases | 39Ar | mean residence time of middle-aged groundwater (some centuries) |
Origin and authenticitiy of our food determine the quality and price. To verify the authenticity of food and food additives isotope analysis is widely used. Isotope methods are often the only possibility to prove cases of food fraud. They can provide the following information:
- Geographical origin of products
- Verification of illegal or non-declared additives (e.g. water, sugar, acids) or parental material
- Differentiation of naturally or synthetically produced commodity
- Control of organic or conventional growth
MEDIUM |
ISOTOPE SYSTEM |
INFORMATION |
| food and food additives | δ13C | origin of food; verification of illegal additives; authenticity |
| food and food additives | δ18O, δ2H | origin |
| food and food additives | δ15N | sort of growth of fruit and vegetable - organic or not organic? |
| food and food additives | δ34S | origin |
| food and food additives | δ87Sr | origin |
| food | δ11B | sort of growth of fruit and vegetable - organic or not organic? |
| food and food additives | 134Cs,137Cs, 90Sr, 239Pt, 131I, 129I | radiological evaluation (e.g. influence of nuclear accident) |
Organic pollutants such as HCH and BTEX have a strong impact on groundwater. It is often difficult to find the person or company responsible for the contamination. The determination of the carbon isotope ratios of the organic pollutants allows the assignment of the contamination to the originator as well as a qualitative and quantitative estimation of the biological degradation (natural attenuation).
Medium |
ISOTOPE SYSTEM |
INFORMATION |
| organic pollutants | δ13C of CHC, BTEX, PAH δ2H, δ37Cl of CHC |
Person or company responsible for the contamination; natural attenuation; age of contamination |
Isotope methods are important tools to evaluate the genesis of crude gas and oil. They allow the exploration of possible reservoirs near to the surface. The investigation of isotopes from hydrocarbons gains information about the genetic mechanisms of e.g. methane and the maturity of higher hydrocarbons.
In the course of the energy turnaround and the climate protection sustainable resources should replace fossil energy sources. The determination of the radioactive 14C verifies the amount of the renewable part in a product (e.g. synthetics, biogenic lubricants, oils, etc.). It is also possible to analyse the exhaust fumes from e.g. a biomass heating power plant for its fraction of renewable resources which are used in the combustion.
Medium |
ISOTOPE SYSTEM |
INFORMATION |
| crude gas | δ13C and δ2H of CH4 and higher carbon | origin and genesis of hydrocarbons; maturity of reservoirs; possible crude gas and oil reservoirs |
| bed rock, shales |
δ13C and δ2H of CH4 and higher carbon; |
origin and genesis of hydrocarbons; maturity of reservoirs; possible crude gas and oil reservoirs |
| crude oil |
δ13C and δ2H of oil components and single coal compounds |
maturity of oil reservoirs; genesis of reservoirs |
| renewable resources |
δ13C, 14C of organic material |
determination of biogenic carbon fraction; origin and composition |
| flue gas |
14C of flue gas (adsorber) |
determination of biogenic carbon fraction |
| secondary combustible materials, sludge, biogas, secondary raw material |
14C of organic material, biomass and CO2 |
determination of biogenic carbon fraction |
| paint, varnishes, synthetic materials, glues, lubricants etc. | 14C of organic material, biomass and CO2 | determination of biogenic carbon fraction |